WHY CHOOSE US
选择我们的理由
服务不及时
等额退
服务不满意
免费送
服务不满意
全额赔款

服务不及时
等额退

服务不满意
免费送

服务不满意
全额赔款
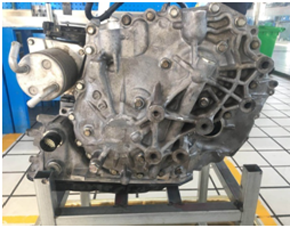
公司自动变速箱产品
集变速箱总成维修、保养、油品销售,技术培训于一体,解决自动变速箱疑难问题中有着独到的技术和视角
公司自动变速箱产品
集变速箱总成维修、保养、油品销售,技术培训于一体,解决自动变速箱疑难问题中有着独到的技术和视角

ABOUT US
关于我们

相信品牌力量 相信知名企业

企业使命:
★创新维修技术
★解决各项变速箱疑难故障
★建立行业维修标准
★促进行业健康发展
企业愿景:
★成为中国自动变速箱维修领域里具有影响力的品牌
★ 让每个员工过上有尊严的生活
企业价值观:
勤奋 诚恳 严谨 创新
NEWS CENTER
CONTACT US
电话:
网址: